Exploring the Versatility of ELISA: From Techniques to Applications
Neomycin ELISA Kit: Detecting Traces of the Antibiotic with Precision
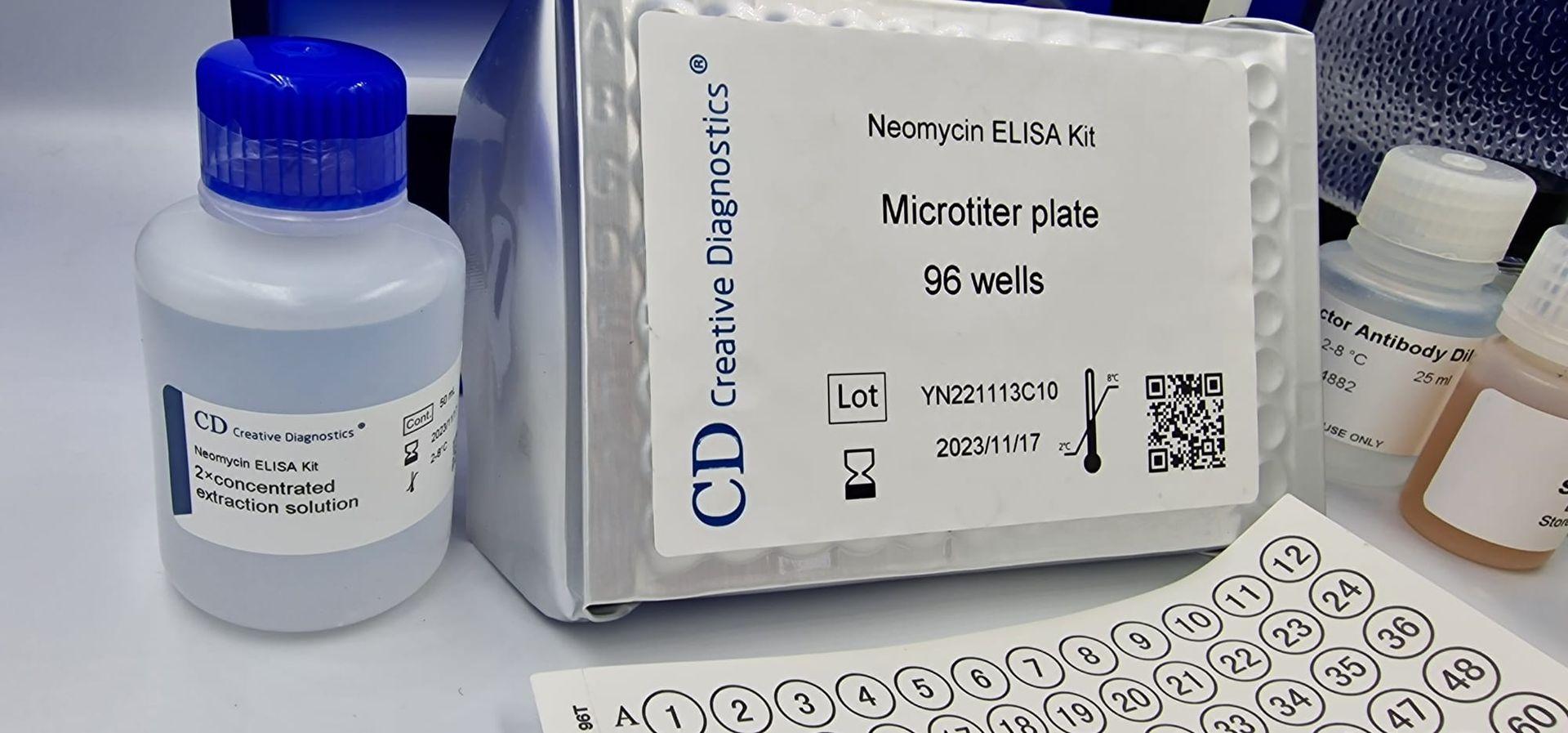
The Neomycin ELISA kit is a powerful tool for researchers and scientists seeking to detect the presence of neomycin in various samples. This type of enzyme-linked immunosorbent assay (ELISA) offers a sensitive, specific, and quantitative method for analyzing neomycin, an antibiotic commonly used in veterinary medicine and sometimes found in food products.
Unveiling the Kit's Components:
At the heart of the Neomycin ELISA kit lies a microtiter plate. Imagine a plate with 96 tiny wells, each acting as a miniature reaction chamber. These wells hold the key players of the assay:
- Samples: Milk, tissue sections, cell culture supernatants, or other matrices suspected to contain neomycin.
- Neomycin-specific antibodies: These molecules bind to neomycin with high affinity, forming the basis for detection.
- Enzyme conjugate: An enzyme linked to another neomycin-specific antibody, amplifying the signal upon binding.
- Substrates and chromogens: These chemicals react with the enzyme conjugate, producing a colored product if neomycin is present.
The ELISA Dance:

The Neomycin ELISA kit follows a well-defined choreography:
- Sample loading: The suspected samples are added to designated wells on the microtiter plate.
- Antibody incubation: Neomycin-specific antibodies are introduced, allowing them to bind to any neomycin present in the samples.
- Washing: Unbound antibodies are removed, leaving only those attached to neomycin.
- Enzyme conjugate addition: The enzyme-linked antibody is introduced, binding to the neomycin-antibody complexes.
- Another wash: Unbound enzyme conjugate is eliminated.
- Substrate and chromogen addition: These chemicals react with the bound enzyme conjugate, producing a colored product.
- Colorimetric reading: The intensity of the color, measured using a microplate reader, is proportional to the amount of neomycin in the sample.
Advantages of the Neomycin ELISA Kit:

- High sensitivity: The kit can detect minute amounts of neomycin, even in complex matrices.
- Specificity: The antibodies specifically target neomycin, minimizing interference from other substances.
- Quantitative results: The color intensity directly reflects the neomycin concentration, allowing for precise quantification.
- Fast and convenient: The entire assay can be completed within a few hours, making it a time-efficient tool.
- Wide applicability: The kit can be used for various sample types, offering versatility in research and analysis.
Applications of Neomycin ELISA Kit:

- Monitoring antibiotic residues: Ensuring food safety by detecting neomycin residues in milk, meat, and other food products.
- Pharmacokinetic studies: Tracking the absorption, distribution, and elimination of neomycin in animals and humans.
- Environmental monitoring: Assessing neomycin contamination in water and soil samples.
- Research on antibiotic resistance: Investigating the presence of neomycin-resistant bacteria.
By employing the Neomycin ELISA kit, researchers and scientists gain a valuable tool for uncovering the presence and quantity of this important antibiotic in various contexts. This information plays a crucial role in ensuring food safety, understanding antibiotic use, and advancing research in related fields.
Introduction
Enzyme-Linked Immunosorbent Assay (ELISA) stands as a cornerstone in the field of immunoassays, enabling researchers to detect and quantify various analytes with high specificity and sensitivity. This article delves into the diverse applications of ELISA, the intricacies of its technique, fundamental principles, different types, required reagents, and the production and utilization of mouse monoclonal antibodies in IgE ELISA assays.
Understanding ELISA Technique:
ELISA is a widely used biochemical technique that relies on the specific binding between an antigen and an antibody. Its fundamental principle involves immobilizing the target antigen onto a solid surface, such as a microplate, followed by the addition of an enzyme-linked antibody that recognizes the antigen. The enzymatic reaction generates a measurable signal, usually a color change, indicating the presence and quantity of the target analyte.
Types of ELISA:
There are several variations of ELISA tailored to specific applications, including direct, indirect, sandwich, and competitive ELISA. Each type offers unique advantages in terms of sensitivity, specificity, and versatility, catering to diverse research needs.
Reagents and Protocol:
Performing an ELISA assay requires a series of reagents including coating buffers, blocking agents, primary antibodies, enzyme-conjugated secondary antibodies, substrates, and stop solutions. The protocol typically involves coating the microplate wells with the antigen, blocking nonspecific binding sites, incubating with primary and secondary antibodies, developing the enzymatic reaction, and measuring the signal intensity.
Production and Purification of Mouse Monoclonal Antibodies:
Mouse monoclonal antibodies are valuable tools in ELISA assays, particularly for detecting specific analytes like IgE. These antibodies are produced by immunizing mice with the target antigen, isolating antibody-producing B cells, and fusing them with myeloma cells to generate hybridomas. The resulting hybridoma cells are screened for antibody production, and positive clones are expanded and cultured to harvest antibodies. Purification techniques such as affinity chromatography are employed to obtain highly pure monoclonal antibodies for use in ELISA assays.
Utilization in IgE ELISA:
ELISA assays targeting immunoglobulin E (IgE) are crucial for diagnosing allergic conditions and monitoring immune responses. Mouse monoclonal antibodies specific to IgE are used in sandwich ELISA formats to detect and quantify IgE levels in biological samples. These assays provide valuable insights into allergic sensitization, disease progression, and therapeutic efficacy.
Conclusion:
ELISA remains a versatile and indispensable tool in biomedical research, offering a robust platform for various applications including disease diagnosis, drug development, and basic science investigations. With continuous advancements in technology and methodology, ELISA continues to evolve, further expanding its utility in diverse fields of study.
ELISA as a Biological Assay:
Beyond its applications in clinical diagnostics and research, ELISA serves as a fundamental technique in pharmaceutical and biotechnology industries. It plays a crucial role in drug discovery, where it is used to screen compound libraries for potential drug candidates, assess drug efficacy, and monitor biomarker levels in preclinical and clinical studies.
Furthermore, ELISA finds utility in agricultural and environmental sciences for detecting contaminants, pathogens, and toxins in food, water, and soil samples. Its high sensitivity and specificity make it an invaluable tool for ensuring food safety, environmental monitoring, and quality control in various industries.
The advent of automated ELISA systems has further streamlined assay procedures, enabling high-throughput screening and analysis with improved accuracy and reproducibility. These advancements have significantly enhanced the efficiency and reliability of ELISA, making it a preferred choice for a wide range of applications across different disciplines.
In conclusion, ELISA continues to play a pivotal role in biomedical research, clinical diagnostics, and industrial applications due to its versatility, sensitivity, and ease of use. As technology advances and new methodologies emerge, ELISA is poised to remain at the forefront of immunoassay techniques, driving innovation and enabling groundbreaking discoveries in various fields of science and medicine.
Understanding the Mechanism of Enzyme-Linked Immunosorbent Assay (ELISA)
Enzyme-Linked Immunosorbent Assay (ELISA) stands as a cornerstone in biomedical research, clinical diagnostics, and pharmaceutical development due to its precision, sensitivity, and versatility. At its core, ELISA operates on the principle of specific antigen-antibody interactions coupled with enzymatic amplification, enabling the detection and quantification of target molecules within biological samples.
The technique begins with the immobilization of the target antigen onto a solid surface, typically the wells of a microtiter plate. This process, known as coating, ensures that the antigen firmly adheres to the surface, ready for subsequent interactions.
Following coating, any unbound surface of the microtiter plate is blocked using a blocking agent such as bovine serum albumin (BSA) or milk protein. This step prevents nonspecific binding of antibodies and minimizes background noise, enhancing the assay's specificity and accuracy.
Next, the sample containing the target antigen is added to the coated wells and allowed to incubate. If the antigen is present in the sample, it binds specifically to the immobilized antibody on the microtiter plate, forming an antigen-antibody complex.
After an incubation period, the wells are thoroughly washed to remove any unbound or nonspecifically bound components from the sample, further ensuring the specificity of the assay.

To detect the antigen-antibody complex, a secondary antibody linked to an enzyme, such as horseradish peroxidase (HRP) or alkaline phosphatase (AP), is added to the wells. This secondary antibody recognizes and binds specifically to the antigen-antibody complex formed in the previous step.
Following another washing step to remove excess secondary antibody, a substrate specific to the enzyme conjugated to the secondary antibody is added to the wells. The enzyme catalyzes a reaction with the substrate, resulting in the production of a detectable signal, often a color change or luminescence.

The intensity of the signal generated is directly proportional to the amount of antigen present in the sample. This allows for the quantification of the target antigen's concentration in the sample, providing valuable insights into various biological processes, disease states, and drug responses.
In summary, ELISA's mechanism relies on the precise interplay between antigens, antibodies, enzymes, and substrates to detect and quantify target molecules with high sensitivity and specificity. Its robustness and ease of use make ELISA a fundamental tool in modern laboratory practice, facilitating a wide range of applications across multiple disciplines.
Furthermore, ELISA's versatility extends beyond traditional laboratory settings, finding applications in point-of-care testing, epidemiological studies, and environmental monitoring. Portable ELISA kits are being developed for rapid detection of infectious diseases, biomarkers, and environmental contaminants in resource-limited settings. These portable devices offer quick and reliable results, making them invaluable tools for disease surveillance, outbreak management, and public health interventions in remote areas and developing countries.

Moreover, the advent of automated ELISA systems has revolutionized assay procedures, enabling high-throughput screening and analysis with improved accuracy and reproducibility. These advancements have significantly enhanced the efficiency and reliability of ELISA, making it a preferred choice for a wide range of applications across different disciplines.
In addition to its applications in research and clinical diagnostics, ELISA plays a crucial role in quality control and validation processes in various industries, including pharmaceuticals, biotechnology, and food safety. It is utilized for batch testing of pharmaceutical products, detection of contaminants in food and beverages, and monitoring of environmental pollutants, ensuring compliance with regulatory standards and consumer safety.

Overall, ELISA's widespread adoption and continuous innovation underscore its significance as a cornerstone technique in biomedical research, clinical diagnostics, industrial applications, and public health initiatives. As technology advances and new methodologies emerge, ELISA is poised to remain indispensable in advancing our understanding of diseases, improving healthcare delivery, and addressing global health challenges.
Enzyme-Linked Immunosorbent Assay (ELISA) stands as a pivotal technique in contemporary biomedical research, clinical diagnostics, and pharmaceutical development. Renowned for its precision, sensitivity, and adaptability, ELISA operates on the principle of specific antigen-antibody interactions coupled with enzymatic amplification, allowing for the detection and quantification of target molecules within complex biological matrices.
ELISA commences with the immobilization of the target antigen onto a solid surface, typically the wells of a microtiter plate. This process, known as coating, ensures the secure attachment of the antigen, providing a stable foundation for subsequent assay steps. Following coating, the unbound surface of the microtiter plate is blocked using a blocking agent, such as bovine serum albumin (BSA) or milk protein, to prevent nonspecific binding and minimize background interference, thereby enhancing assay specificity.
Subsequently, the sample containing the target antigen is introduced into the coated wells and allowed to incubate. If the antigen is present within the sample, it specifically binds to the immobilized antibody on the microtiter plate, forming an antigen-antibody complex. After an appropriate incubation period, the wells are thoroughly washed to remove any unbound or nonspecifically bound components, further ensuring the accuracy and specificity of the assay.

To detect the antigen-antibody complex, a secondary antibody conjugated to an enzyme, such as horseradish peroxidase (HRP) or alkaline phosphatase (AP), is introduced into the wells. This secondary antibody recognizes and binds specifically to the antigen-antibody complex, facilitating the enzymatic reaction that follows.
Following another washing step to remove excess secondary antibody, a substrate specific to the enzyme conjugated to the secondary antibody is added to the wells. The enzyme catalyzes a reaction with the substrate, resulting in the generation of a detectable signal, typically a color change or luminescence, directly proportional to the amount of antigen present in the sample. This signal can then be quantified using a spectrophotometer or plate reader, enabling precise determination of the target antigen's concentration within the sample.

Beyond its utility in basic research and clinical diagnostics, ELISA finds application in diverse fields, including pharmaceutical development, environmental monitoring, and food safety assessment. Multiplex ELISA platforms enable simultaneous detection of multiple analytes within a single sample, providing comprehensive insights into complex biological processes and disease states. Moreover, ELISA plays a pivotal role in vaccine development and quality control, aiding in the quantification of antigen concentrations, assessment of antibody responses, and evaluation of vaccine efficacy.
In the realm of personalized medicine, ELISA assists clinicians in predicting disease progression, selecting optimal therapies, and monitoring treatment responses by providing quantitative data on biomarker levels. Additionally, ELISA serves as a vital tool in environmental monitoring, enabling the detection of contaminants, pathogens, and toxins in various matrices, contributing to public health interventions and environmental stewardship.
In summary, ELISA's versatility, sensitivity, and reliability render it an indispensable tool across a spectrum of scientific disciplines and applications. As research continues to advance and technological innovations emerge, ELISA remains at the forefront of scientific progress, driving discoveries that enhance human health and well-being.